Marketing agency targets poor interns for GSK paid trials

GraduateFog.co.uk, a website for UK university graduates, said it had been approached by Touchpoint Digital, a marketing agency, asking to publish an article encouraging readers to consider clinical trials as an “immediate income to tide you over during the coming months.”
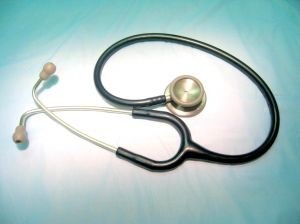
Patients receiving therapeutic benefit from trials are not remunerated, but “healthy volunteers” are paid for participation in clinical studies at a maximum of £2,000 per trial, four times a year, as the since published article pointed out.
The article suggested clinical trials as an alternative to part-time work for young people to support themselves during unpaid internships.
The student website said it was “uncomfortable with the idea of presenting participation as a simple solution to the financial problems many of our young readers are facing, as you struggle with high unemployment, zero-hours contracts, low wages and sky-high cost of living.”
Science writer Ben Goldacre described the language being used in the article as “crass and exploitative” and added, “We can only wonder how many more similar stories have been lost beneath the radar,” reported GraduateFog.
Other articles encouraging students or recent graduates to join paid clinical trials have been published on student websites Studential and Scape Living, marked as written by either GSK Clinical Trials or by Touchpoint Digital. Both suggested paid volunteering as a quick-fix money solution and provided a link to GSK’s volunteer site, with one saying “Up to £8000 per year is a reasonable-sized sigh of relief when the credit card interest begins to bite.”
GSK response
When in-Pharmatechnologist.com contacted GSK, their spokesman David Daley told in-Pharmatechnologist.com Touchpoint Digital had been responsible for the placement of the articles, and “their tone was wrong” and focused “very heavily on payment, and actually we wouldn’t recruit solely on the basis of payment.”
GSK added the language “trivialises the role of clinical trials in developing new medicines and the part our volunteers play in that process. This isn’t acceptable and we’re looking into what happened.
“Clinical trials are important in helping us develop new medicines. It is important that we find the right volunteers and it is right that they are paid for their time. But we need to talk about trials in a serious way and be thoughtful about volunteers’ contributions and how their actions help advance medical research.”
GSK is no longer working with the marketing agency, Daley told us.
The European Medicines Agency, which is responsible for ensuring application of good clinical practice (GCP) told us before a clinical trial can be conducted in the EU, it must receive approval from the national competent authority as well as a positive opinion from the ethics committee.
Directive 2001/20/EC Article 6 of the European Parliament requires the ethics committee to give an opinion on “the amounts and, where appropriate, the arrangements for rewarding or compensating investigators and trial subjects” and “the arrangements for the recruitment of subjects.”
The Guideline for Good Clinical Practice from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)states that one of the responsibilities of the ethics committee is to review “subject recruitment procedures (e.g. advertisements).”