Latest FDA COVID-19-related advice and actions
According to NPR, as of this week, 246m doses of the various vaccines against COVID-19 have been distributed in the US. More than 105m Americans have been fully vaccinated, or nearly 32% of the total population.
However, the pandemic is far from over, and US Food and Drug Administration (FDA) measures to monitor treatments and vaccines, watch out for fraudulent products, advise life-sciences professionals on dealing with restrictions, and issue (or revoke) emergency use authorizations (EUAs) continues. Here are the latest actions and advice from the agency.
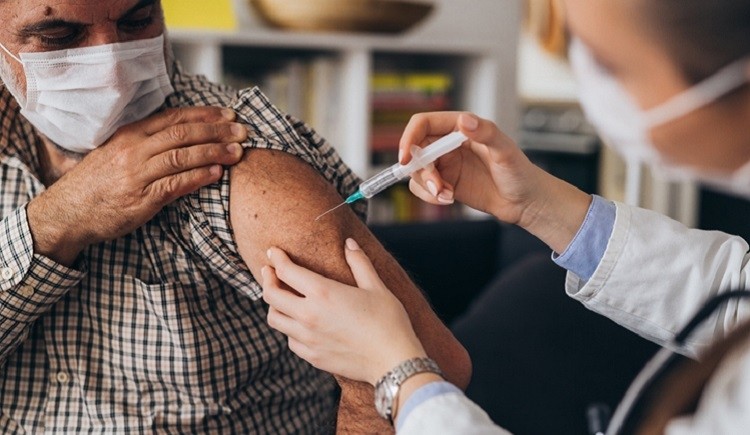
J&J vaccine pause lifted
The FDA and Centers for Disease Control and Prevention (CDC) issued a joint statement that administering the use of the Janssen/Johnson & Johnson COVID-19 could resume, after a safety review. The agency had recommended stopping the use of the vaccine after concerns due to a limited number of adverse events.
In response to concerns about the safety of the vaccine, the agency expanded content on a frequently asked questions page. The topics on the page include reasons behind the decision to pause, then resume vaccine distribution; types of adverse events reported; and side effects.
Antibody EUA revoked
The agency has revoked the EUA for the monoclonal antibody bamlanivimab, which had been granted to Eli Lilly in November for the treatment of mild-to-moderate COVID-19 cases. According to DA officials, the agency has determined the known and potential benefits of the treatment (administered alone) no longer outweigh the known and potential risks for its authorized use, especially in the face of resistant variants.
“Other monoclonal antibody therapies authorized for emergency use remain appropriate treatment choices when used in accordance with the authorized labeling and can help keep high-risk patients with COVID-19 out of the hospital,” said Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research. “We urge the American public to seek out these therapies when needed while we continue to use the best data available to provide patients with safe and effective treatments during this pandemic.”
Remote facility evaluation guidance
The FDA has released Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities During the COVID-19 Public Health Emergency. The document outlines a range of interactive and virtual tools that could be of use as industry professionals grapple with the challenges associated with conducting inspections in the face of pandemic-related shutdowns and travel restrictions.
The FDA’s acting commissioner Janet Woodcock said in a statement that continuing inspections despite obstacles is important to help keep Americans safe.
“We recognize that remote interactive evaluations do not replace inspections, and that there are situations where only an inspection is appropriate based on risk and history of compliance with FDA regulations," said Woodcock. “Within the exceptional context of a global pandemic, we see remote interactive evaluations as part of a necessary strategy to evaluate medical product facilities by using all available approaches to ensure the medical products we regulate are safe, effective and of high quality.”
Unauthorized COVID-19 treatments
The FDA issued a warning letter to the owners of the website PharmacyGeoff.md for marketing unapproved drugs for multiple diseases, including COVID-19. The agency recommends consumers be wary of buying drugs online, check out its BeSafeRx resources for advice on how to safely buy medicines off the internet, and consult with healthcare providers before doing so.
The agency also issued a warning letter to Group Cyrenne Inc. (dba HomeoAnimal) for selling a Virus Defense Kit reportedly intended to prevent, treat or cure COVID-19 in companion animals. Additionally, the company was chided for marketing unapproved pet drugs for cancer, epilepsy, heartworm, parvovirus, and anemia.