Indian government suspends EU trade talks over spat with GVK Biosciences

The proposal from India to defer the talks on Aug. 28 between the chief negotiators of India and the EU as part of the Broadbased Investment and Trade Agreement comes as the European Commission has officially banned the sale of more than 700 drugs tied to clinical trials run by GVK.
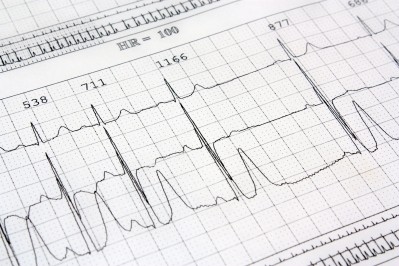
The ban followed inspections by French regulators that revealed data manipulations of electrocardiograms (ECGs) during the conduct of some studies of generic medicines for more than five years, according to the EMA.
In a statement, the Indian government said it’s “disappointed and concerned by the action of EU in imposing legally binding ban on the sale of around 700 pharma products clinically tested by GVK Biosciences, Hyderabad. The Government has engaged on the issue with various EU regulators over past 8 months.”
Although the government notice didn’t address the data manipulations cited by the French regulators, it did point out: “It is pertinent to mention that most of these drugs are already in EU market for many years without any adverse pharmacovigilance report from any member state.”
EMA’s Committee for Medicinal Products for Human Use (CHMP) previously said there is no evidence of harm or lack of effectiveness linked to the conduct of studies by GVK Biosciences at Hyderabad. Some of these medicines may remain on the market in some countries if they are of critical importance for patients because alternatives cannot meet patients’ needs.
Here’s an updated list of the medicines that fall under the ban.