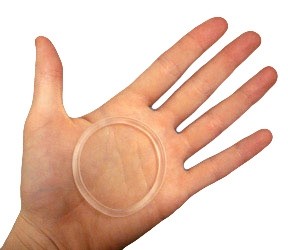
Particle Sciences completes manufacture of intravaginal ring

The device is a hollow tube designed to deliver tenofovir disoproxil fumarate (TDF) through the vaginal lining to prevent infection by human immunodeficiency virus (HIV). It has already shown efficacy in primate trials and, now the manufacture of trial supplies is complete, it is poised to begin the next stage of development and enter first-in-human studies.
Mark Mitchnick, CEO of Particle Sciences, told Outsourcing-Pharma.com his company had been selected to provide “manufacturing and quality to the project and has just completed the manufacture of the ring for clinical trials.”
The project is being led by the Translational Prevention Research Center at Albert Einstein College of Medicine and is funded by the National Institutes of Health (NIH), with the ring itself being designed by Patrick Kiser, Associate Professor of Biomedical Engineering at Northwestern University.
“The consortium selected us,” Mitchnick added, as “Particle Sciences has a long history with prevention technologies and has manufactured a number of other rings that have gone into clinical trials.”
Furthermore, he said it would be likely the company would be involved in further development of the ring.
Though normally the firm designs its own drug delivery devices, he said in this case the ring is a new design. “Therefore there were atypical processes that had to be scaled to make this venture viable, something PS does very well.”
The ring’s design attracts vaginal fluid into the core of the device, solubilizing some of the drug allowing it to be released, the designer Kiser told our sister publication in-Pharmatechnologist.com last week.
“Other polymers used in IVR design are not capable of releasing TDF because the drug is not soluble in them,” he said, adding: “We use a water swellable elastomer in which the drug TDF has appreciable solubility.”
The device could potentially be used to administer a number of other women’s health drugs and preventative therapies.