CRO market in Asia to see 'significant increase' through 2020

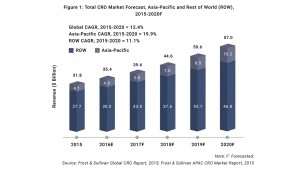
According to the report, published by Frost & Sullivan, the global contract research organization (CRO) market is expected to grow at a compound annual growth rate (CAGR) of 12.4% to reach $57bn in 2020, up from $31.8bn in 2015.
Notably, during this period the Asia Pacific CRO market is estimated to grow at a CAGR of 19.9%, as opposed a 10.4% CAGR expected in North America.
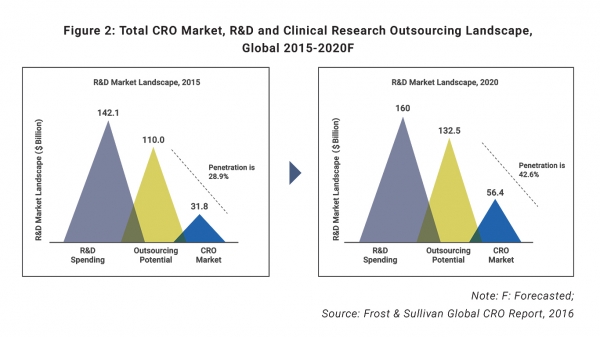
Increased R&D activity and a shift toward outsourcing are key drivers of this growth, according the report, with the potential to outsource more than three-quarters of R&D spending by biotechnology and pharmaceutical companies.
As such, the report explained a “significant increase in the CRO market” is expected, growth that will be catalyzed by increased outsourcing, as penetration is expected to increase from 28.9% in 2015 to nearly 43% in 2020.
Speed, quality, and costs
According to the report, three key factors make Asia a preferred destination for clinical trials: speed, worldwide data acceptability, and cost effectiveness.
John Moller, CEO of the Australia-based CRO Novotech, explained speed is realized during patient recruitment – with access to “huge, treatment-naive patient populations, in very large hospitals,” he told us.
Specifically, a pool of approximately 4.0bn people, with more than 2.0bn in urban areas.
According to the report, Asian countries are also beginning to show similar or higher disease incidence rates to Western nations, which provides a comparable environment to conduct clinical trials.
The area also provides comparable quality and is regularly audited by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
“The percentage of critical EMA Good Clinical Practice (GCP) inspections in the Asia-Pacific region was less than in other regions, and the percentage of major findings was among the lowest,” the report explained.
The percentage of official actions taken in FDA inspections was also lower than North America.
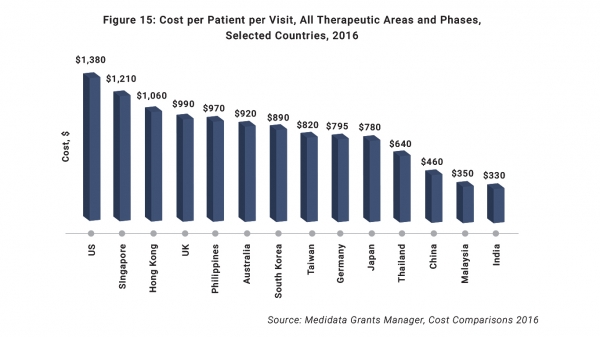
“Finally, costs in Asia are highly competitive, and our clients see savings of 30 to 40% savings versus the US and Europe,” concluded Moller.
An evolution
“The market has evolved rapidly over the last five years,” Moller explained, adding that this evolution has been driven by two main trends.
“First the increase in wealth of Asian economies has led to an increased focus on health by consumers, and hence a greater engagement in the advantages of clinical trials,” Moller said.
This wealth has also led to investments in hospital and clinical trial infrastructure.
“Governments in Asia have shown incredible foresight in recognizing that developing a supportive clinical trial environment will have positive long term implications for the development of scientific talent and a vibrant Biopharmaceutical industry,” he added.
According to Moller, many countries in Asia have explicitly identified clinical trials as political and economic priority areas.
“We see many hospital groups engaging with us to develop standardized contracts, while others are pooling patient data to help with recruitment,” he explained. “Regulators are shifting sequential processes into faster parallel processes, and KPIs are being developed by a number of groups focused on improving turnaround times.”
The next five years
Moller expects the current trends to accelerate over the next five years.
“Penetration of clinical trials in Asia remains low and so there is plenty of capacity to maintain the outperformance that we have seen,” he said. “Asia is a diverse region and the differences in governments and cultures actually encourages a level of healthy competition in clinical trial development.”
Additionally, Moller predicted that as one country implements an innovative initiative, other countries will adopt and improve the most successful ones.
Over time, this will “continue to benefit sponsors and patients,” he said.
However, the biggest changes will be in India and China, as both governments are implementing significant policy changes to improve quality and reduce regulatory timeframes.
As these changes evolve, Moller explained, easier access to patients "will have massive implications.”