FDA strengthens disqualify & debar actions
Some members of Congress have been critical of the FDA’s use of disbarment and disqualification powers, in particular claiming that the agency has been slow to remove individuals from the drug development process.
The US Government Accountability Office (GAO) has been asked to perform a review of the FDA’s operations but in the meantime the agency has acted independently.
Debarment and disqualification procedures have now been revamped after the FDA’s internal review concluded that it needed to act quicker to ensure the safety of clinical trial participants.
These changes include increased staffing and centralised coordination. In particular the FDA has designated an administrative law judge as a presiding officer and has assigned the good clinical practice programme to oversee the disqualification process.
By implementing these changes the FDA believes that its actions will be more rapid, transparent and consistent.
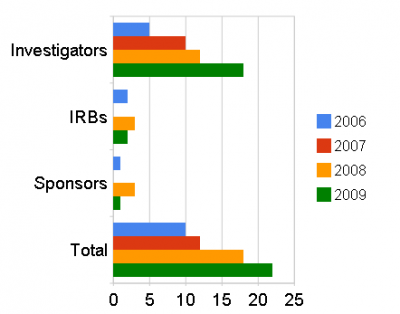
Evidence of this has emerged in the short time that the measures have been in place. The FDA began debarring clinical trial investigators in 1992 and averaged two or three actions a year.
This rate has increased significantly since October 2008. Since October the FDA has initiated debarment actions against five individuals, debarred two others and begun internally processing a further nine procedures.
Furthermore, the agency claims that the time taken to process each action has been reduced significantly. In the past debarment could occur three or four years after the date of conviction but this has been reduced to within one year.
The FDA has also revised its website to provide complete lists of disqualified and disbarred investigators.