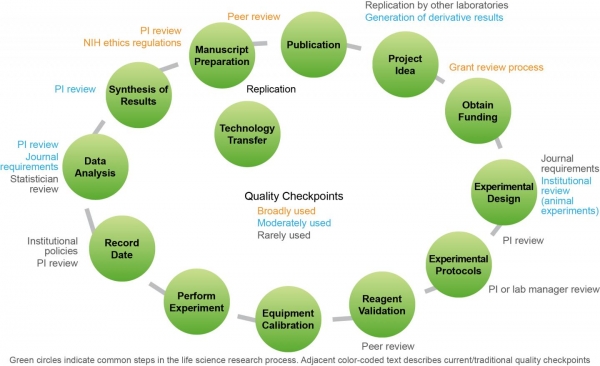
GBSI report outlines steps to improve preclinical research reproducibility

According to the Global Biological Standards Institute (GBSI), the Reproducibility2020 Report: Progress and Priorities outlines “encouraging steps taken thus far by the community,” and includes strategies and actions that individuals and organizations can join.
Leonard P. Freedman, PhD, President at GBSI told us, of the actions identified in the report, the importance of funders and journals leveraging initiatives by other stakeholders is paramount.
“Their guidelines and initiatives are proving useful at this early stage of the community-wide effort,” he said. “Many exemplary actions are underway by the life science research community, its policymakers and funders.”
Documenting the reproducibility problem
According to GBSI, the reproducibility problem came to light in 2012 when Amgen scientists reported they were able to reproduce only six of 53 landmark preclinical cancer biology studies.
A year later, the institute released the “Case for Standards,” which was one of the first comprehensive reports to address the irreproducible biomedical research.
The report drew attention to issues, such as reporting and publication bias, underpowered studies, lack of open access to methods and data, and lack of clearly defined standards and guidelines.
Subsequently, in 2015, GBSI completed an economic study and reported the frequency of irreproducible preclinical research exceeded 50% – with associated annual costs of approximately $28bn in the US.
“Since then, the community has come quite far in a relatively few number of years to reach a point where each stakeholder group has begun working on new policies and innovative solutions,” Freedman said.
“Much work still needs to be done, and a culture change in academic research needs to occur.”
Strategies outlined by GBSI
- Drive quality and ensure greater accountability through strengthened journal and funder policies.
- Engage the research community in establishing community-accepted standards and guidelines in specific scientific areas.
- Create widely accessible online training and proficiency testing.
- Enhance open access to data and methodologies.
“More actions are needed in the area of rethinking incentives in academia to de-tangle the pressure points that generate low reproducibility rates,” explained Freedman.
“The long-standing cloud that hangs over the academic researcher to publish or perish is one that will require the academic sector to take a deep dive into the future of promotions and tenure that presently are influenced by success in publishing findings and obtaining significant funding.”
Working together
GBSI is currently leading a community-wide effort to develop standardized guidelines for validating research antibodies. It is also developing proficiency testing and training sessions with funding provided in part by NIH.
“The research community is full of talented, motivated people who care deeply about producing high-quality science,” added Freedman.
"We are optimistic about the potential to improve reproducibility, and working together to solve a very complex problem that is integral to the development of translatable discoveries that lead to new treatments and cures.”