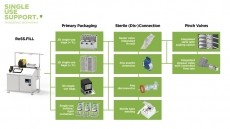
Drug delivery, formulation
42 Results
Suppliers
Follow us
Product Innovations
Increasing the Bioavailability of Oncology Drugs
Oral tyrosine kinase inhibitors (TKIs) are a class of cancer drugs that can be highly susceptible to issues with solubility in the gastrointestinal tract
Let your Senses Guide you into the world of Art & Science: Meet with Funtrition & Sofgen during Supply Side West 2023
Procaps Group, a leading integrated LatAm healthcare and pharmaceutical services company,
Addressing Challenges with Clinical In-Use Testing
Lonza Drug Product expert Léa Sorret PhD explores Clinical In-Use Testing of Biotherapeutics in this white paper. Léa shares her expertise and describes...