Pharma finance
Medical device CROs: the next growth opportunity?
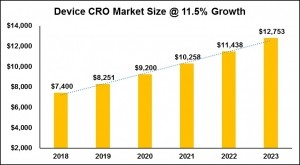
The medical device CRO market is expected to continue growing at 11.5% per year, according to a recent report by Grand view Research. At this rate, the market could reach nearly $13bn by 2023. (See figure 1).
However, currently there doesn't appear to be a true mid-size device CRO (greater than $50m in device revenue), and this market void could be a significant value creation opportunity.
Obviously, 11.5% is a high growth rate and a lack of industry funding or a slowdown in outsourcing adoption could make these projections overly optimistic.
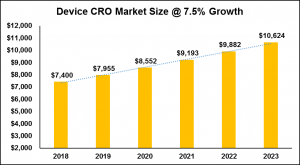
So, I ran the numbers again using a 7.5% growth rate (see figure 2) which is within the range of expected growth for pharmaceutical outsourcing. Probably conservative, but still pretty compelling.
Outsourcing penetration
Device companies are not as far along the outsourcing curve as pharmaceutical and biotech companies.
However, the good news is that as device outsourcing strategies mature, the market could more than double before reaching current pharma penetration levels – which makes a 2023 market estimate between $10.6bn and $13.3bn realistic.
Medical device trial growth
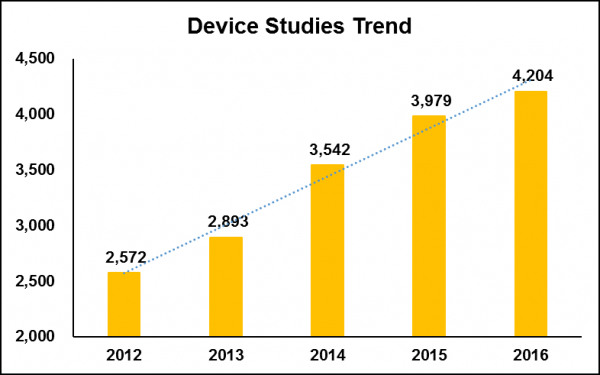
According to trial data on ClinicalTrials.gov, device trials have increased 63% since 2012 – and this number will likely grow once 2017 is reported in full, as there is a delay in reported trials (see figure 3).
Global expertise needed
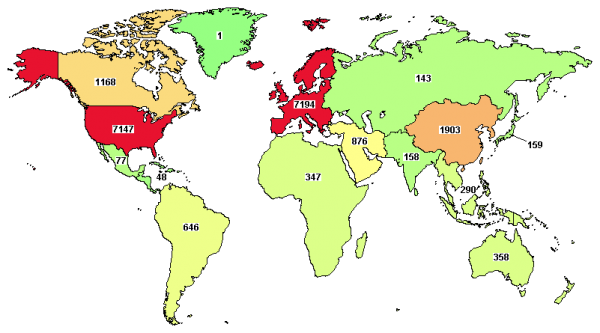
Below is a graphic from ClinicalTrials.gov that shows the geographic distribution of all device studies reported from 2011 through 2017. As you can see, a majority of trials are run in Europe and the US, with many in Asia as well.
As the industry grows, regulations evolve, and reimbursement strategies become more complex, a medical device CRO with global capabilities and expertise will be in demand.
New large entrants and evolving technology
The rapid advancement of both mobile and sensor technology has enabled technology giants such as Google and Microsoft to enter the device market.
Additionally, Apple has filed a patent that could suggest medical device aspirations and we know it is also on the lookout for MedTech talent.
Increased competition, advancing technology and a blurring of the line between traditional technology and medical devices will increase the need for device expertise.
What’s next?
I can see several paths toward the development of several mid-size device CROs. I think the first is that the device business units within larger CROs grow at a rate faster than the market, and are run quasi-independently from the pharmaceutical business units.
Device trials differ from pharma trials, so that may be a necessity regardless.
A second passive approach would be organic growth from some of the larger, independent device CROs in the space.
Lastly, I believe a very likely path forward is consolidation within the sector, with either the larger CROs or private equity acting as a financial sponsor. However, consolidation will be tricky as there aren’t many platforms to purchase, so integration risk may be high.
Though, the market projections, along with financial synergies, may make the risk worthwhile. Either way, this will be an interesting market to track over the next several years.
Jason Monteleone is a guest contributor to Outsourcing-Pharma.com’s Pharma Finance column.Jason is the president of Pivotal Financial Consulting, LLC, which provides divestiture assistance, acquisition advisory services and strategic planning to the pharmaceutical outsourcing industry. He was previously CFO at Theorem Clinical Research and Omnicare Clinical Research and senior finance director at MDS Pharma Services.Contact Jason at wzbagryrbar@cigsvanapr.pbz and follow him on Twitter @JMPivotal.