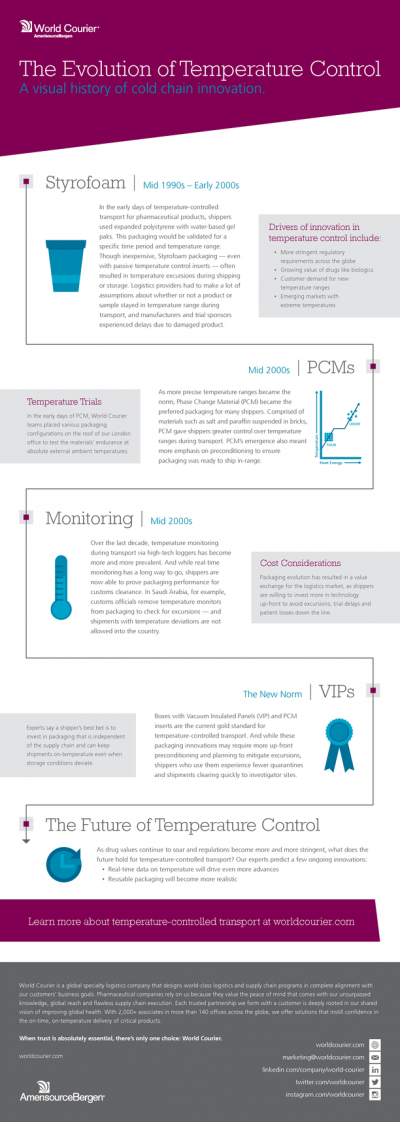
Promotional Features
Risk in global logistics: the top overlooked factors
There is risk inherent to global pharmaceutical logistics. When designing a logistics strategy any pharmaceutical company or shipper should make certain they consider the following risk factors:
- Temperature control
- Speed of service
- Transport handling
- Customs clearance
However, within these broad factors, there are many aspects that could be overlooked. These include:
Road and air transportation of the packaging
Organizations can misjudge the difficulty of executing transport. Significant preparatory work is required to ensure that the physical movement of the shipment is handled smoothly, including planning the route, selecting an airline and the type of temperature-controlled packaging. Equally important is providing proper instructions to airlines and ground handlers, and ensuring that all documentation meets custom requirements to safeguard against any delays in clearance.
Customs clearance
The complexity of custom clearances in areas such as Southeast Asia, Latin America and Africa can be underestimated. It’s widely assumed that the shipment will be available within hours anywhere in the world, but that is not the case. Conducting due diligence on the customs requirements for documents to avoid clearance delays is essential. Companies must implement procedures in collaboration with their specialty logistics partner for all shipments requiring customs clearance, verifying all relevant paperwork is comprehensively available and correct ahead of the actual transport to ensure smooth customs clearance and avoid delays.
Preparing and handling of temperature controlled packaging
The design of new temperature controlled units simplifies preparation and handling. Such packaging improves the security of the product but does not negate the risk of improper handling; a risk some companies may underestimate. The emphasis is now on ensuring that all the preparatory work is done correctly. Companies need to develop processes including training, testing of equipment and labelling.
Underestimation of environment during pickup and delivery
Critically, with temperature-controlled shipments, a significant number of issues occur during loading of the product into the packaging or unloading the goods at the final destination. Proper preconditioning of the packaging system requires professional equipment and training of the handling staff. The packing process should occur in a temperature-controlled environment to avoid temperature excursions at the earliest and final stages of the transport chain. At the point of delivery, they should unload wherever possible in a temperature controlled environment, and provide instructions to ensure proper storage conditions at the final consignee site.
The risk of using consignee brokers
There are frequent reports of incidents of temperature deviations which occurred due to improper handling by consignee brokers. To avoid these scenarios, the brokers and the pharmaceutical company’s logistics partner need to collaborate closely to ensure compliance with the handling instructions. Ideally, the logistics partner should have direct access to the shipment.
Language barriers
Cultural complexities are a reality of global logistics. Ensuring the success of a logistics strategy requires consistent and precise language and communications skills. Introducing interpretation into communications ranging from work instructions, regulatory paperwork, meetings and telephone conversations increases the risk of miscommunication. All parties must be able to communicate in the local language and understand the unique vernacular and cultural variances of an area to ensure all instructions are fully understood and followed.
Integrity of the business partner
All key stakeholders of the pharmaceutical supply chain must commit to the highest ethical standards and recognize the importance of all applicable laws. In addition to compliance with GDP guidelines, vendors must avoid violations of international anti-bribery laws, including the US Foreign Corrupt Practices Act (FCPA) and the UK Bribery Act. Proper due diligence processes as well as a sophisticated vendor management program should be part of company policy to mitigate the risk of non-compliance as well to ensure that all business dealings are conducted with the highest level of business ethics, honesty and integrity.
Pharmaceutical companies must focus on developing a robust risk management strategy in the mobile supply chain, ensuring that effective controls and procedures are in place to proactively mitigate the potential risks.