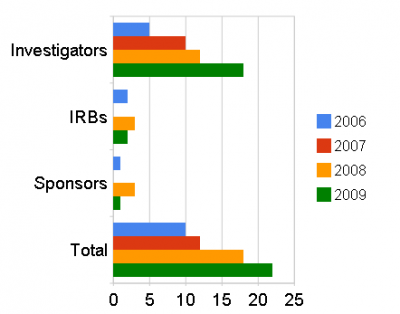
Ignore a form 483? Not wise say FDA
A form 483: “Lists observations made by the US Food and Drug Administration (FDA) representative during the inspection of your facility. They are inspectional observations, and do not represent a final agency determination regarding your compliance.”
Responding to this is not compulsory but in a recent presentation the FDA warned that doing nothing increases the likelihood of receiving a warning letter.
A warning letter will not be issued if the FDA is satisfied that a company has adequately corrected the deficiencies highlighted in the form 483, which can be achieved by responding to the initial contact.
In addition the receipt of a response indicates to the FDA that a company understands and acknowledges the observations, is willing to comply with the agency and is a credible business.
To ensure that the response achieves these goals the FDA recommends that responses asses each observation separately, stating whether the company agrees or disagrees with each.
For each observation that the company deems to be valid a complete and realistic corrective action plan, detailing implementation timeframes, should be included in the response. Whenever it is feasible documentation detailing corrective actions should be included.
The root-cause of each problem should also be considered and everything should be framed with the relevant regulatory requirements in mind.
In summary the FDA believes that “a well-reasoned, complete, and timely 483 response is in your best interest”.
The complete presentation can be found here.