InDex and Parexel trial for Ulcerative colitis won't be in US due to high cost

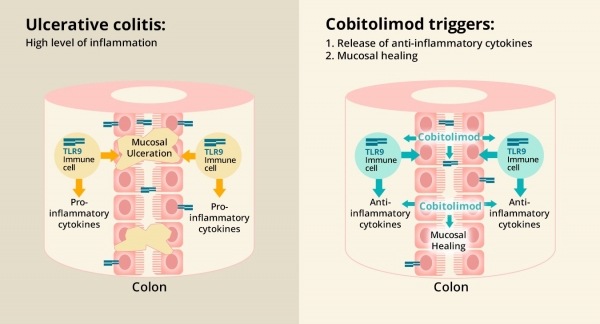
Based in Stockholm, Sweden, InDex’s CONDUCT study will evaluate a topically applied drug cobitolimod for moderate to severe active ulcerative colitis.
Cobitolimod is a rectal formulation of a DNA-based ImmunoModeulatory Sequence, administered locally in the patient’s large intestine using an enema. The drug works as a topical anti-inflammatory to help the gut lining heal.
The study, to be managed by the contract research organisation Parexel, will enrol 215 patients in centres across Czech Republic, France, Germany, Hungary, Italy, Poland, Romania, Russia, Serbia, Spain, Sweden and Ukraine.
However, although the global CRO Parexel is headquartered in Massachusetts, InDex will no longer be conducting the trial in US-based centres “due to the high cost per patient.”
Peter Zerhouni, CEO of InDex Pharmaceuticals said “We are now working together to advance the CONDUCT study as efficiently and quickly as possible.”
Higher costs
Conducting clinical trials in the US has been getting more expensive, with most costs being down to administrative staff, site monitoring and clinical procedural operations.
Zerhouni told Outsourcing-Pharma that InDex had originally planned to carry out the trial in both the EU and US, with up to 20 patients (10%) at a site C centre in the US. But this had been a challenge regarding budgets.
Zerhouni explained “On top of delivery costs from having a central lab in Europe we would have to transport US samples to, it is also more expensive recruiting patients in the US – you have to pay them more.”
Therefore, despite the US also having a significant economic burden for the irritable-bowel disease, InDex has decided not to run the trial in the states.
“We decided it would be a better option to include some additional European countries in the CONDUCT study, which was a change from the original plan,” he added.
Partnering for Phase III
“Our intention is to find a big partner who would run the Phase III in the US. At the moment it's way too early to know who, but Parexel is a big and reputable CRO which has done a lot of trials in this therapeutic area," Zerhouni told us.
He explained: “We have already been working with Parexel under a startup running agreement for about a year as we planned the trial.”
“The only difference now is that we had to switch to a full contract as we enrol our first patient.”
The plan is to commence enrolment by end of Q2 this year.