EU safety logo set to tighten net on dodgy food supplement sales

European-wide efforts to guide consumers when buying products online will come into effect in June this year with the introduction of a mandatory safety logo to appear on every page – and not just the homepage – of online pharmacies.
When consumers click on the logo they are forwarded to the national regulatory authority’s homepage where they can check if the pharmacy has been verified. This prevents rogue companies from simply copying and pasting the logo onto their site.
According to the communications chief at the Austrian Health Agency (AGES), Christoph Baumgaertel: “This in the end will enable consumers to recognise at first sight if they are shopping at a certified legal homepage with legal, authorised products or at an illegal store. So, in consequence if you don’t have the logo and are not registered in the list, you are by law an illegal online-pharmacy.”
Toxic promises
Last week AGES issued a warning against US-based Miracle Mineral Solutions' MMS and MMS2 - toxic supplements it said are chemically equivalent to industrial-strength bleach - bringing Austria in line with other European countries such as the UK and Switzerland, that have spoken out against the products.
MMS is founded by US Archbishop Jim Humble, although the company webpage lists no fixed address and scientific backing is characterised as, 'scientific clinical trials conducted in a prison in Malawi.'
MMS claimed the supplements are “the answer to AIDS, hepatitis A, B and C, malaria, herpes, TB, most cancer and many more of mankind's worse diseases,” but tried to differentiate the two by noting MMS is 28% sodium chlorite while MMS2 is 70% calcium hypochlorite.
Both products come with a citric acid activator solution which, when mixed, produces chlorine dioxide, “a toxic gas with a pungent chlorine-like odour which is used as a bleaching agent,” according to AGES.
The supplements have been known to cause severe nausea, vomiting and diarrhoea, potentially leading to dehydration and reduced blood pressure when taken as directed. If diluted less than instructed they can damage the gut and red blood cells, potentially resulting in respiratory failure.
But Baumgaertel noted no adverse events had been reported in Austria.
Danish warning
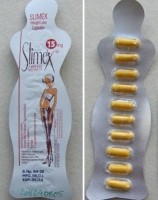
Meanwhile the Danish Health and Medicine Authority has warned against slimming products, Slimex 15 and Slimex 15 New Formula, which contain sibutramine, a drug which suppresses the appetite by affecting neurotransmitters.
In 2010 all products containing sibutramine were withdrawn from the Danish market due to side effects including severe cardiovascular reactions and brain haemmorrhages and it has provoked numerous supplement busts across the EU in recent years.