Chocolate drug delivery has NIH interest, Lycotec CEO says

The firm is now carrying out trials using chocolate to deliver its Lycosome tech – lycophene reverse micelles - designed to protect the transportation of cargo molecules through the gastrointestinal tract to deliver amphiphilic compounds to the liver.
In Phase IIa studies, the platform was proven to improve the efficacy of statins – specifically with cholesterol lowering simvastatin – four-fold, and of anti-inflammatory protein and peptide molecules over 100-fold.
CEO Ivan Petyaev told us the formulation is not limited to just proteins and statins, and that it could technically be used to boost patient compliance for any oral compound.
Speaking to in-PharmaTechnologist.com, he said: “We can have powdered and tableted forms, of course. But the idea of taking little pieces of chocolate is really good for longer administration. We can have a more convenient form to deliver for longer-term diseases.”
Petyaev also said that the formulation could also mean greater success in paediatric drug delivery, adding: “Make people enjoy taking their drugs and they will do it.”
Lycotec is now working on other formulations for non-chocaholics, including a system that can be dispersed in water, and is pondering a system similar to the probiotic yoghurt shots that are currently popular in the food industry.
“The pharma industry is already working with a number of programmes that can be delivered in novel ways,” he added. “This is a new industry.”
However, though previous studies have touched on food-based delivery – for instance the University of Illinois’ tomato paste Lycopene – he said the method is still relatively unexplored, and believes Lycotec is the first to use chocolate.
The firm is now looking for partners to further develop the technology. “We just need to find companies looking for new innovation,” he said.
Asked whether he felt the industry will embrace such a novel idea, he replied: “I discussed with the FDA and they welcomed it – were relieved even. They’re looking for new technologies because of a need for better control, both of delivery and of patient compliance.”
How it works
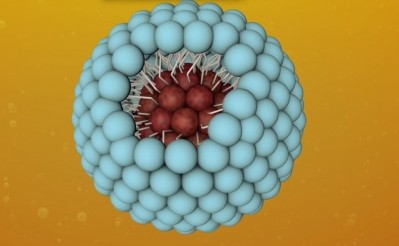
The Lycosome platform is able to pass relatively unscathed through the gastrointestinal system because high carotenoid molecules, like trans-lycopene or lutein, do not have digestive enzymes in the gut, and so get absorbed in unmodified crystals.
The molecules are also “attracted” to liver tissues – or hepatocytes – because they have more than 10-100 fold lycopene and other carotenoids binding receptors than any other tissues.
“This means that lycopene carrier would preferably and therefore foremost be able to deliver its cargo molecules to the liver, and concentrate them at a higher level than in other organs,” said Petyaev.
He added that both the formulation and the tech itself would open up doors for the pharma industry, saying: “For companies looking to improve drugs coming off patent, this could be an answer.
“It also opens up opportunities for new drugs and new molecules that couldn’t be developed before because they couldn’t pass the peptides and proteins through the GI.”