Guest column
Clinical research speaks up: Showing government leaders what the work really means

For most people outside of clinical research, clinical trials are invisible. The industry uses language that is unfamiliar, employs tools that are highly technical and specialized, and works in locations that include the familiar (hospitals and clinics) and the obscure (labs and data centers.)
So it can be difficult to clearly show government officials, in real and tangible ways, the essential work that helps to advance medical innovations that change lives.
Despite our relative anonymity, the clinical research industry is big, global and growing. In the past decade, work done by clinical research organizations (CROs) has increased by 40%, and is expected to total $45 billion by 2022.
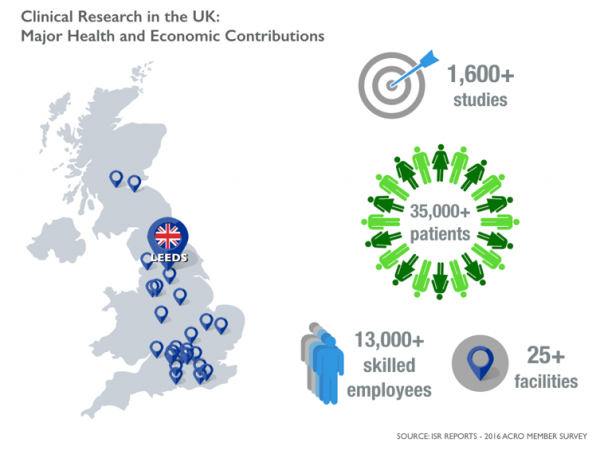
In the UK in 2015, clinical research touched the lives of more than 35,000 patients and employed over 13,000 highly skilled people. Those are huge economic and health impacts. Yet policymakers are often not familiar with our industry, our work, or how lives are changed. Until now.
In 2018, clinical research is speaking up. The Association of Clinical Research Organizations (ACRO) represents the companies on the front lines of clinical trials and medical innovation. We want to show government leaders what this work really means. How do we start? Let’s make it real.
On April 13 in Leeds, UK, ACRO convened senior clinical leaders from member companies to host Hilary Benn, the Member of Parliament for Leeds Central. We wanted to illuminate the research happening in his backyard.
At a clinical research site operated by Covance, we talked about the fundamentals of clinical trials, what this important work means for the people of the community and 300 facility employees, and how clinical research companies in the UK are preparing to meet the challenges brought on by Brexit. The implications go far beyond the community and even the UK.
“It was an honor to host the Rt Hon Hilary Benn and leaders from the R&D community at our clinic in Leeds, where we have provided research opportunities to healthy volunteers and patients for more than 30 years,” said John Ratliff, CEO of Covance.
“The CRO industry is home to some of the most talented and dedicated researchers, not just in the U.K. but around the world. Together, we have supported major medical breakthroughs and advances in scientific knowledge. Drug development is a global enterprise in which the U.K. has historically played a vital role. This meeting was a tremendous opportunity to examine the opportunities and challenges related to Brexit from the CRO perspective and, more importantly, for patients.”
With more than 25 research facilities and over 100 clinical trial sites in the UK alone among ACRO member companies, the conversation is just beginning.
This year, in communities around the UK, ACRO will convene additional discussions with government officials and the wide array of stakeholders who are connected to clinical research.
Around the world, medical innovations reach patients because of the essential clinical trial process. And this work couldn’t be completed without the patients and highly skilled people in places like Leeds.
ACRO is excited to partner with its members to bring greater visibility to clinical research and raise the volume of conversation about an exciting future.