Recipharm in talks to make LIDDS' prostate cancer drug-device candidate
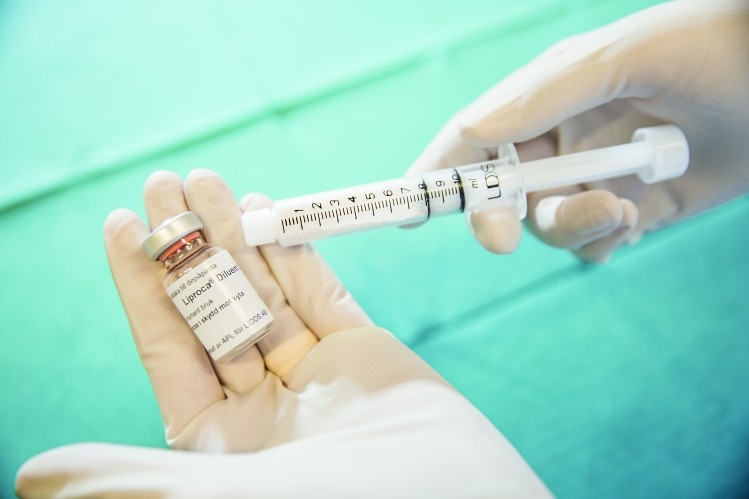
Helsingborg, Sweden-based LIDDS (Local Intelligent Drug Delivery System) is developing a potential treatment for prostate cancer using its proprietary drug delivery platform.
Liproca Depot comprises of a modified-release formulation of 2-hydroxyflutamid, prepared as a paste, delivered in a pre-filled container/syringe, which also acts as mixing equipment of the paste prior to injection.
Currently in Phase II clinical trials, LIDDS is now looking for a contract manufacturing organisation (CMO) for the scale-up, manufacture and quality assurance of the product and fellow Swedish firm Recipharm says it has begun negotiations to be that partner.
If successful, Recipharm General Manager Maria Lundberg told Outsourcing-Pharma.com production would take place in its facility in Solna, Sweden, to begin with.
“When this platform is implemented, it can be used for more substances and we will be there to support Lidds with their future plans,” she said. “This is new technology and specialized equipment is needed. We will need to invest but not expand our facility.”
But the deal is not guaranteed, Lundberg added, saying she was unaware which other manufacturers – if any – have submitted a tender offer. “This is a very interesting product opportunity, which should be of interest to many CDMOs.”
LIDDS CEO Monica Walter was unable to divulge who else the firm was speaking to and the timelines involved in selecting a partner.
However, she did say LIDDS was looking for a “trusted company with technical capabilities and – depending on clinical trial results – the commercial manufacturing capacity.”
LIDDS holds the IP on the Liproca Depot product, and says it holds a number of advantages over current treatments for prostate cancer.
These include fewer side-effects due to the local administration, reduced dosing frequency and improved patient compliance, and an increased accuracy in dose-positioning due to the injected material being visible with ultrasound.
























