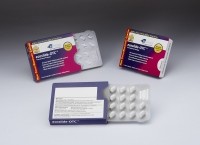
Child-resistant blister packaging gets a redesign

The designer and cGMP manufacturer of paperboard packaging solutions has launched Ecoslide-OTC, a child-resistant paperboard pack for blister-packaged over-the-counter (OTC) medication.
“Not much has changed since child-resistant blisters for medicine were introduced in 1972,” Ward Smith, director of marketing and business development at Keystone Folding Box Co. told Outsourcing-Pharma.com.
As Smith explained, child resistant blister packs commonly use a heavy gauge lidding material – which he said consumers often find hard to push through.
“By transferring the child-resistant feature away from the foil lidding and onto the reclosable locking carton, the consumer has a much easier time removing the tablet from the blister,” he said.
The new design was molded after the Ecoslide-RX package, which launched in 2012.
According to Smith, the locking mechanism used in both designs keeps the blister from being accessed by children and retains the blister at the edge of the carton. “This ensures that branding information and product instructions remain with the medication,” he said.
Additionally, by transferring the child-resistant feature to the carton, Smith said the size of the blister can be reduced, which in turn, reduces the amount of film and foil needed.
“This allows for a more compact package size that conveniently fits in a pocket or purse. The reduced size also allows for more product to be merchandized on retail shelves, and offers savings in shipping and distribution,” he explained.
Per its name, the package also uses 100% recyclable paperboard and doesn’t require additional plastic or film laminates. “This eco-friendliness fits well into the sustainability objectives of many manufacturers and major retailers,” said Smith.
When asked what the initial feedback has been, Smith said several manufacturers are considering how they can best use the pack soon.