Fujifilm building $37m facility to support liposome drugs
The 4bn yen ($37m) facility will be built at a site in Toyama, japan run by Toyama Chemicals, a subsidiary of Fujifilm Corporation.
“The first purpose [of the plant] is the manufacturing of [our] own liposome drugs, including FF-10832 which will start clinical trial in the US this year,” Fujifilm spokesperson Kana Matsumoto told this publication.
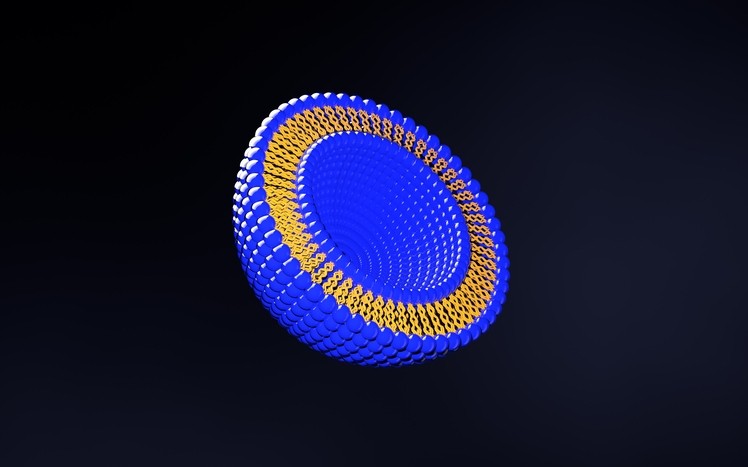
Liposomes, or artificially constructed vesicles, are made from the same organic phospholipids that make up cell and bio membranes and can be designed to deliver active pharmaceutical ingredients (APIs) to specific areas.
Toyama’s FF-10832 consists of an anti-cancer drug Gemzar (gemcitabine), developed by Eli Lilly for the treatment of pancreatic cancer, formulated with liposomes with a uniform size of approximately 80nm.
But “Fujifilm will also collaborate with other companies for offering the liposome technology,” Matsumoto told us. “Fujifilm has advanced liposome technology, cultivated through photographic film business, and the production facility will be designed and developed by Fujifilm.”
She added the facility will make liposome drugs in compliance with international GMP standards, and will use Fujifilm’s Information and communication Technologies (ICT), including “centralised management of various data such as production process, quality evaluation and operation data.”
Construction of the new facility will begin in September, and operations are expected to begin in February 2020.




















