CRO supporting genomics-based opioid study designed to improve addiction treatment

The program is a collaboration between Kalamazoo Community Mental Health and Substance Abuse Services (KCMHSAS), Ferris State University College of Pharmacy, and Genemarkers, a Kalamazoo, MI-based contract research organization (CRO).
“The three firms have a positive working relationship and have collaborated on other projects in the past,” said Anna Langerveld, PhD, founder of Genemarkers.
Genemarkers specializes in genomics and performs pharmacogenomics (PGX) testing at its CLIA certified lab. As part of the program, the CRO will be performing PGX testing and genetic analysis with a novel addiction risk panel.
“The approach of this study is aligned with precision medicine initiatives and is a first step in extending precision medicine into clinical practice for addiction – from prevention through recovery services,” Langerveld told Outsourcing-Pharma.com.
Study design
The study is designed to determine if PGX testing can improve treatment response to opioids for chronic pain and medication-assisted treatment for opioid addiction. It also aims to identify genetic markers of addiction – which could support initial opioid prescribing and potentially prevent addiction in the future, Langerveld said.
Clinical researchers at Ferris State University and KCMHSAS will analyze study data to better understand “clinically actionable recommendations that could be used to optimize medication treatment of the patients enrolled in the program,” according to the firms.
Study recruitment is underway and is expected to be completed by the end of the summer, with data analysis expected by mid-Fall, Langerveld explained. In total, 400 participants will be enrolled, 200 for addiction and 200 for chronic pain.
“The next steps would be to expand the study and recruit additional patients to further validate the results,” she added. “Discussions are underway for follow-on work with health care systems in the Midwest that serve at-risk patient populations.”
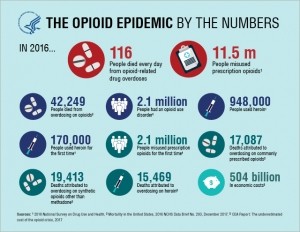
The Opioid Crisis
The opioid crisis was declared a national Public Health Emergency under federal law on October 26, 2017, after an estimated 116 people died every day from opioid-related drug overdoses in 2016. Forty percent of these deaths involved a prescription opioid.
According to the Centers for Disease Control and Prevention, the total economic burden of prescription opioid misuse in the US is $78.5bn a year.
The opioid study being conducted in collaboration with Genemarkers is made possible by funding provided by the Michigan Department of Health and Human Services (MDHHS), which recently secured $16.3m in federal funding through the State Targeted Response to the Opioid Crisis Grant.
Additionally, State Representative Brandt Iden (R-Oshtemo Twp.) advocated for $750,000 in funding through supplemental appropriations specifically focused on preventing opioid abuse and addiction.